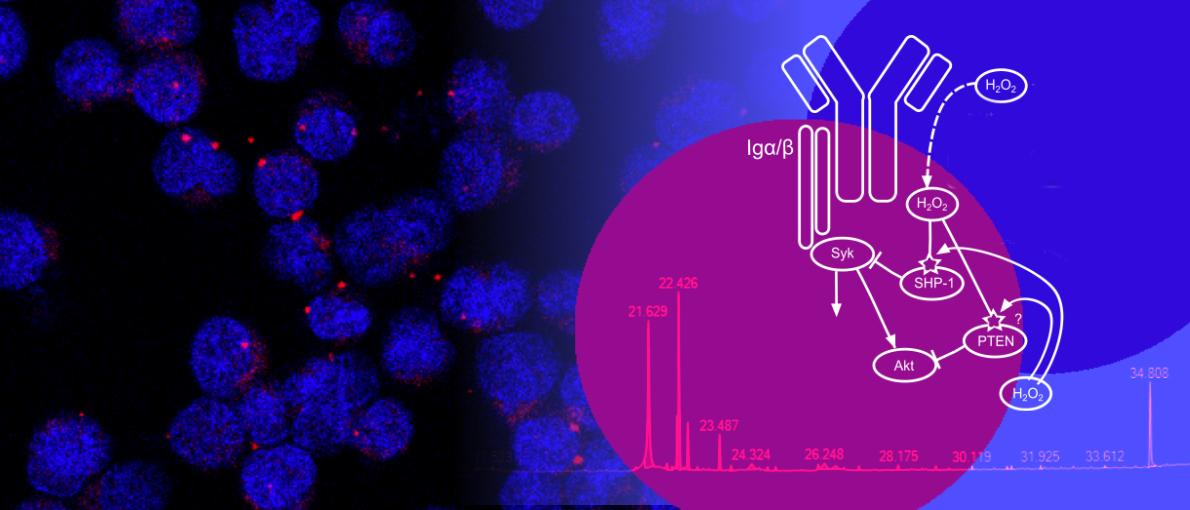
The overarching goal of our research is to elucidate how metabolism and signal transduction are integrated in B cells in the normal and diseased state. B cells play a crucial role in our immune responses to pathogens. Reduced B cell activity can thus result in increased sensitivity to infections. On the other hand, aberrantly activated B cells can contribute to the development of various autoimmune disorders or can give rise to malignancies.
In the past, research has focused primarily on understanding how protein-based signaling networks govern B cell fate and function. However, recent years have put forward the view that the metabolic program of immune cells is not simply a side product of cellular activation but is delicately interwoven with protein-based signaling and actively steers cell fate decisions. Our aim is to define how different signaling pathways shape B cell metabolism, how B cells cope with metabolic stress and how the metabolic microenvironment affects signal transduction. We have shown that aberrantly increased metabolic activity can boost B cell proliferation or render B cells more prone to apoptosis. The choice between these two functionally opposing outcomes depends on the signaling context, the developmental status and the microenvironment of the cells. Moreover, we have shown that production of immunoglobulins can shape metabolic flexibility by governing endoplasmic reticulum homeostasis. We found this function of the immunoglobulins to be independent of their role as cell membrane receptors, thus identifying a novel mode of metabolic regulation in B cells.
In summary, our studies highlight the importance metabolic processes play in orchestrating B cell responses. As cancer cells face increased metabolic demands and are often exposed to hostile environments a better understanding of how cell metabolism is regulated could help to identify new vulnerabilities in malignant B cells and inspire the development of new treatment strategies.











Julia Jellusova studied biology and received her PhD from the Friedrich Alexander University Erlangen-Nuremberg. She completed her PhD on the topic: " Regulation of B1 cells and systemic autoimmunity in mice by Siglec-G" with "summa cum laude" in 2010. Between 2010 and 2015, she worked as a postdoctoral fellow at Sanford Burnham Prebys Medical Discovery Institute in La Jolla, USA. At the end of 2015, she joined the Albert Ludwigs University in Freiburg, Germany, as a junior group leader, working on her habilitation with the support of a Margarete von Wrangell Fellowship. In 2021, she was appointed as a tenure track professor at the Technical University of Munich and focuses her research on signal transduction and cell metabolism of B lymphocytes. In 2023 she became the speaker of the DFG research unit “Crosstalk between B cell metabolism and signaling”.
Further information on the Jellusova lab can be found on the website of the Center for Translational Cancer Research (TranslaTUM)
CD20 as a gatekeeper of the resting state of human B cells.
Kläsener, K., Jellusova, J., Andrieux, G., Salzer, U., Böhler, C., Steiner, S.N., Albinus, J.B., Cavallari, M., Süß, B., Voll, R.E., Boerries, M., Wollscheid, B., Reth, M. (2021). Proc. Natl. Acad. Sci. U. S. A. 118, 1-10. doi 10.1073/pnas.2021342118
Immunoglobulin expression in the endoplasmic reticulum shapes the metabolic fitness of B lymphocytes.
Jumaa, H., Caganova, M., McAllister, E.J., Hoenig, L., He, X., Saltukoglu, D., Brenker, K., Köhler, M., Leben, R., Hauser, A.E., Niesner, R., Rajewsky, K., Reth, M., Jellusova, J. (2020). Life Sci. Alliance 3, 1-17. doi 10.26508/lsa.202000700
Metabolic control of B cell immune responses.
Jellusova, J. (2020). Curr. Opin. Immunol. 63, 21-28. (Review). doi 10.1016/j.coi.2019.11.002
New Methods To Analyze B Cell Immune Responses to Thymus-Dependent Antigen Sheep Red Blood Cells.
McAllister, E.J., Apgar, J.R., Leung, C.R., Rickert, R.C., and Jellusova, J. (2017). J. Immunol. 199, 2998-3003. doi 10.4049/jimmunol.1700454
Gsk3 is a metabolic checkpoint regulator in B cells.
Jellusova, J., Cato, M.H., Apgar, J.R., Ramezani-Rad, P., Leung, C.R., Chen, C., Richardson, A.D., Conner, E.M., Benschop, R.J., Woodgett, J.R., et al. (2017). Nat. Immunol. 18, 303-312. doi 10.1038/ni.3664
Essential Role for Survivin in the Proliferative Expansion of Progenitor and Mature B Cells.
Miletic, A. V.*, Jellusova, J.*, Cato, M.H., Lee, C.R., Baracho, G. V., Conway, E.M., and Rickert, R.C. (2016). J. Immunol. 196, 2195-2204.* equal contribution. doi 10.4049/jimmunol.1501690
Context-Specific BAFF-R Signaling by the NF-κB and PI3K Pathways.
Jellusova, J. *, Miletic, A. V.*, Cato, M.H., Lin, W.W., Hu, Y., Bishop, G.A., Shlomchik, M.J., and Rickert, R.C. (2013). Cell Rep. 5, 1022-1035. * equal contribution. doi 10.1016/j.celrep.2013.10.022
Requirement for Rictor in homeostasis & function of mature B lymphoid cells.
Lee, K., Heffington, L., Jellusova, J., Nam, K.T., Raybuck, A., Cho, S.H., Thomas, J.W., Rickert, R.C., and Boothby, M. (2013). Blood 122, 2369-2379. doi 10.1182/blood-2013-01-477505
Siglec-G Regulates B1 Cell Survival and Selection.
Jellusova, J., Düber, S., Gückel, E., Binder, C.J., Weiss, S., Voll, R., and Nitschke, L. (2010b). J. Immunol. doi 10.4049/jimmunol.1001792
CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity.
Jellusova, J., Wellmann, U., Amann, K., Winkler, T.H., and Nitschke, L. (2010a). J. Immunol. 184, 3618-3627. doi 10.4049/jimmunol.0902711


